In late 2020, drug manufacturer Pfizer had to scale back on its earlier-announced targets for Covid-19 vaccine production when it found that raw materials didn’t meet quality standards for production leading to supply-chain disruptions, worldwide. A few months down the line, in March 2021, public fears in the US were raised when one of the sites for vaccine production at their Mcpherson factory in Kansas, was found to have a longstanding history with contamination. FDA investigators had objected to mold growing on equipment, and even found specks of glass and cardboard in one of the vials of medicine bottled in previous years at this 1970s era facility. This resulted in regulators issuing a warning for the “severe risk posed to patients” by frequent lapses in safety and hygiene, and demanding the immediate recall of the drug in question.
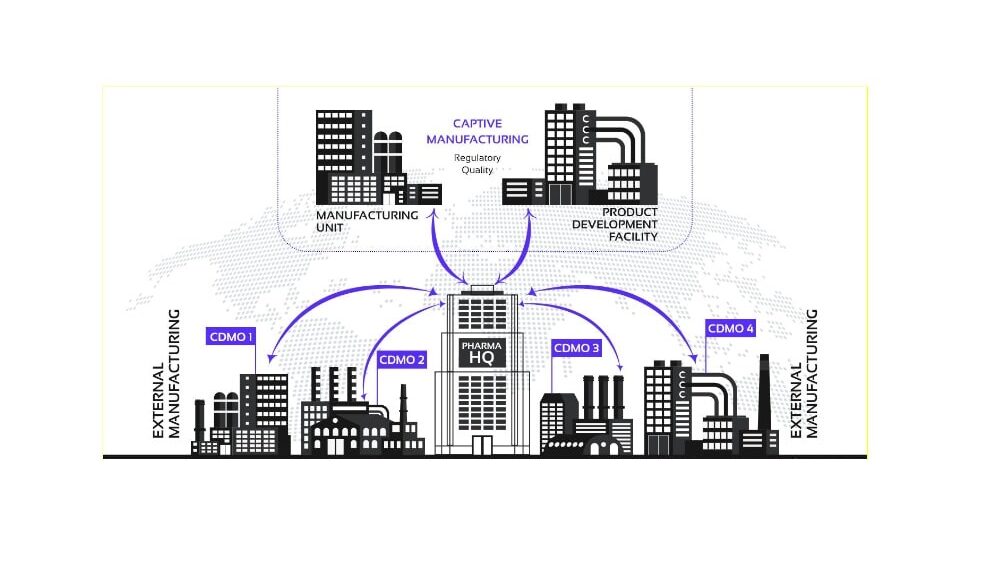
Cases like these are far more common than you would imagine. With more and more pharmaceutical companies turning to third-party contract manufacturers to produce and fulfill orders from across the globe, outsourcing is here to stay. While this distributed model offers economies of scale, it also leads to fragmented communication between various stakeholders. As a consequence, many drugmakers are unable to gather critical-to-quality parameters to be able to take timley corrective action, when issues occur, which in turn has a big impact on product efficacy, safety and regulatory compliance.
Tracing Information Across The Value Chain
Being able to trace provenance, reliably and efficiently, to identify the origin of cases as they occur to swiftly isolate these negative outcomes is becoming more important in the context of our modern-day integrated supply chain landscape.
But supplier ecosystems remain dependent on historical snapshots of information, and there is a need, therefore, to better integrate different stakeholders and enable the exchange of real-time information across today’s complex supply chain. This demonstrated need in the pharmaceutical industry is giving rise to many innovations in the field of data management.
Blockchain makes it easier to connect the dots and close gaps in performance by providing an interoperable system which works across organizational boundaries, and outside corporate walls. Regardless of where a particular drug is manufactured, or where raw material is procured from, brand owners are able to exert greater influence on the final product by ensuring that ingredients meet exacting, global standards by enabling optimum conditions are met every step of the way.
Blockchain Promotes Closer Collaboration Across the Ecosystem
Blockchain is emerging as a preferred technology to facilitate communication between developers of life science products and their multi-tier contract manufacturers.
While SMEs used to rely on manual methods and the MS Office suite to record, store and exchange critical process data via Excel sheets, errors would creep in often. It was difficult, time consuming and resource-intensive to trace problems by collating information located across documents, and large enterprises had their own set of challenges.
With enterprise-wide solutions proving expensive to implement, larger organizations required flexible approaches to suit the dynamic nature of production. Today, however, these pain points are being addressed with collaborative platforms such as those offered by ParamNetwork, which facilitate the exchange of information, more easily, across various nodes in the network.
Blockchain Takes into Considerations around Data Privacy
Keep in mind that sensitivity of data continues to be top of mind within the pharmaceutical sector. And partners are legitimately wary of data changing hands without adequate checks and balances in place. Blockchain offers peace of mind to different stakeholders across the value chain and helps unite downstream and upstream communication, in a permissioned-access manner. This helps promote trust and transparency across the system on account of being permanent, or indelible and hence tamper-proof.
Let’s look at some other specific advantages presented by adopting blockchain-driven collaborative platforms within this sector.
Reducing the burden of regulatory compliance
Compliance pressures in pharma have never been greater, with the FDA issuing more than 200 warnings containing 1,120 citations to prominent drug companies from October 2020 to September 2021 alone. A blockchain-led platform helps mitigate manufacturing risks which could impede compliance, by enabling secure and timely access to documentation across external development and production supply chains.
Pharma companies are able to set specific roles and permissions regarding data entry, transfer, viewing and approvals to align with standard operating procedures or SOPs. Blockchain also makes it possible to retain a complete audit trail of all process changes and data entries making it easier to determine authorship, justify changes and corroborate actions with time stamps which helps establish a persistent product and process data library throughout the manufacturing and distribution lifecycle. When blockchain technology is combined with real-time geolocation and biometric tracking enabled by QR Code serialization, the location and contents of shipments can be traced all the way from the factory to the hands of the end consumers.
Real-Time information to improve on-demand visibility
While pharma companies currently obtain certificates that only capture a moment in time, with blockchain more comprehensive records can be maintained, to authenticate the quality of raw materials or components used across an entire process or batch. Batch records help assess quality in a more consistent fashion, making it easier to spot issues as they crop up and modify downstream operations accordingly. Nowadays, custom tool creators are building on blockchain protocols to turbocharge collaboration by creating low-code Blockchain technology provides clients the advantage of creating custom tools to improve visibility and transparency, to help ERP and QMS (Quality Management System) systems to talk to each other. Moreover, project managers and other stakeholders within the life sciences industry are able to leverage the power of scorecards, dashboards and event management strategies to keep an eye on operations at a glance, and exert greater control.
Technology has the power to help companies accelerate their digital transformation, foster innovation and boost productivity and quality, while cutting down time taken to go to market. Low code workflow builders can be deployed within a few weeks, with an user-centric interface which can be easily configured. What’s more these low code solutions need minimal infrastructure, customization or coding.
This gives companies the flexibility to allow authorized users to start creating process definitions, define steps and set parameters to begin transferring data within hours. These workflow trackers can be connected to other digital data sources via APIs and set up to generate electronic approvals or rejections, target control limits and parameter specification limits for a given batch or shipment.
Interventions such as this have a network effect, helping improve productivity, quality, and reliability of drug manufacture, as well as foster innovation and facilitate regulatory compliance, helping holistically reimagine the pharma supply chain.
About the author:
Vaideeswaran Sethuraman is the founder and CEO Param.Netwok A multi-tier supply chain technology orchestration platform.
⦃param⦄ is built on a proprietary blockchain ledger that enables companies to have a supply chain protocol for enterprise interconnect, data exchange and trust. In simpler terms, ⦃param⦄ enables multi-tier synthesis for knowledge, commerce, and information across n-tier supplier environments for enterprise clients.
Vaidee is a staunch Bangalorean, when he’s not hands-on coding, he loves to travel and drive to untamed places. Fondly called Vaidee or abbreviated as YD on a Starbucks cup of coffee; studied Engineering at BITS and Management from Stanford.