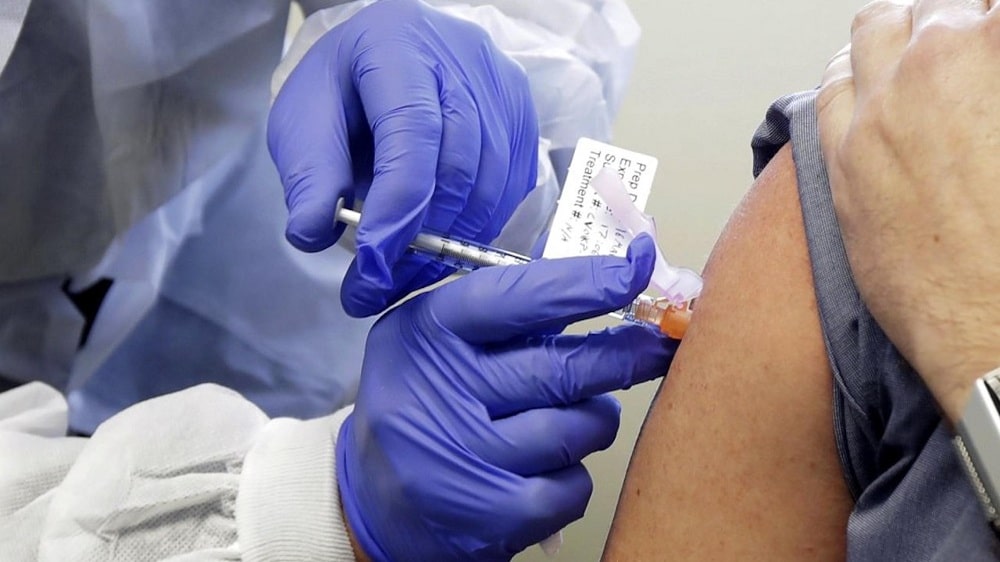
Germany’s vaccines regulator approved live human testing of a potential vaccine against COVID-19 developed by German biotech company BioNTech, the regulator said in a statement on Wednesday (Apr 22).
The trial – only the fourth worldwide – will be conducted on 200 healthy people aged between 18 and 55 in the first stage, said regulatory body Paul-Ehrlich-Institut (PEI).
The preventive agent targeting the virus behind the global pandemic will be tested on more people, including those at higher risk from the disease, in a second stage.
BioNTech said it is developing the vaccine candidate, named BNT162, together with its partner, pharma giant Pfizer.
Advertisement
Tests of the vaccine have also been planned in the United States, once regulatory approval for testing on humans had been secured there.
The trial is a “significant step” in making a vaccine “available as soon as possible”, said PEI.
It added that the approval was the “result of a careful assessment of the potential risk/benefit profile of the vaccine candidate”.
Neither the institute nor the developers specified when the trial will begin, although BioNTech claimed in a statement that it would be “soon” and “ahead of our expectations”.
The institute also said that “further clinical trials of COVID-19 vaccine candidates will start in Germany in the next few months”.